Award-Winning Care

About Our Hospital
Orlando Health Winnie Palmer Hospital for Women & Babies is dedicated exclusively to the unique healthcare needs of women and babies in a caring, family-centered environment. An extraordinary Orlando Health facility, we are home to leading physicians, surgeons and specialists, as well as one of the nation’s largest and most successful neonatal intensive care units.
Patient Care

Labor and Delivery
Labor and Delivery
Learn More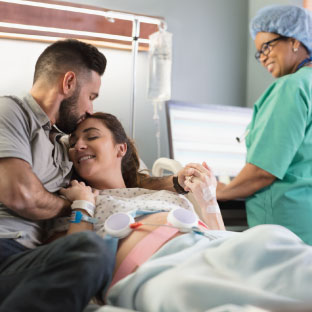
High-Risk Maternity Unit
High-Risk Maternity Unit
Learn More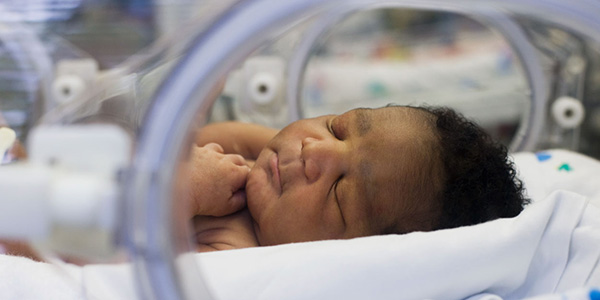
Neonatal Intensive Care Unit
Neonatal Intensive Care Unit
Learn More
Breastfeeding Education
Breastfeeding Education
Breastfeeding is one of the most beneficial things a mother can do for her baby. There are many physical and emotional benefits for both baby and mom.
Learn More
Gynecology
Gynecology
Learn More
Minimally Invasive Surgery
Minimally Invasive Surgery
Learn More
Urogynecology & Pelvic Health
Urogynecology & Pelvic Health
The Orlando Health Women’s Institute Center for Urogynecology offers compassionate care and a complete range of treatments for women with pelvic floor disorders.
Learn More
Counseling Services
Counseling Services
Our licensed counselors are skilled in addressing a variety of questions, concerns and needs that patients and their families may have throughout a patient’s course of care.
Learn More
Orlando Health MyChart
MyChart is a secure, online tool that gives you easy access to all of your Orlando Health information from any of your devices. View your medications, test results and health history. Request an appointment or a prescription renewal. Or send a message to your care team. It’s all there in MyChart.
Choose to Stay in Touch
Sign up to receive the latest health news and trends, wellness & prevention tips, and much more from Orlando Health.










